Endocannabinoid System (ECS): What We Know & How Does It Work?
If you hear the word “endocannabinoid” and immediately think of cannabis, your connection is correct.
While humans have used cannabis for its medicinal and recreational benefits since, well, maybe the beginning of time, the endocannabinoid system remained undetected until the mid-1990s.
Since then, this fascinating and complex system has kept scientists intrigued and excited with its seemingly endless possibilities.
Maybe you’re not in the mood for a science lesson but stick with us while we take a trip through the ECS, how it’s connected to cannabis, and how it could largely impact the future of medicine. It’ll be worth it.
What Is the Endocannabinoid System?
The endocannabinoid system is a complicated and diverse signaling system spread throughout the body. It regulates many biological and physiological processes through endogenous cannabinoid signaling, with the goal of maintaining body homeostasis or balance.
So far, we only know of three parts — cannabinoid receptors (which are G protein coupled receptors), endocannabinoids, and enzymes — but these parts keep almost every bodily system in balance, at least in some aspect.
It’ll require more in-depth testing, but its scope of influence could open the door to many new medicines.
How Does the ECS Work?
The ECS is located almost everywhere in the body, balancing things with a negative feedback loop. It activates the production and release of endocannabinoids that then target specific receptors, letting them know they need to act. Enzymes break down the endocannabinoids.
Here’s a glimpse into each section of the ECS, the mighty role it plays, and why cannabis and its cannabinoids have the effect they do. Later we’ll look at the implications of this.
The following is so oversimplified it almost hurts. For the sake of brevity.
Part 1: The Endocannabinoid Receptors
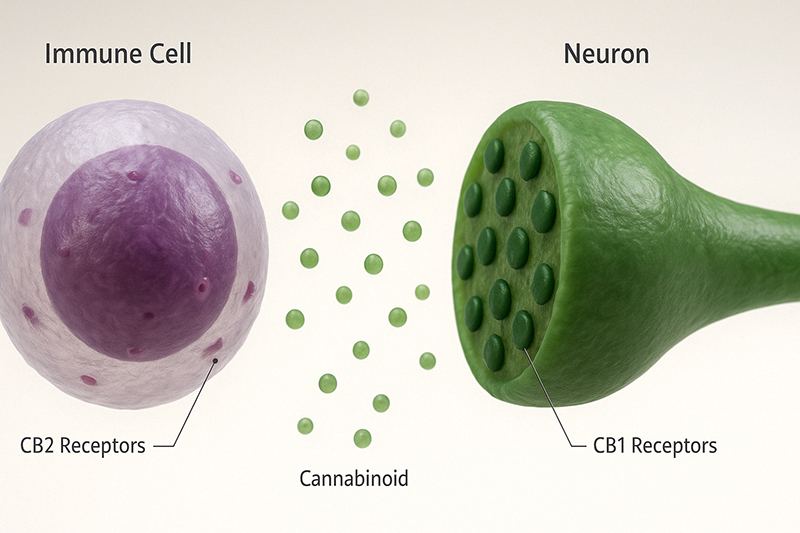
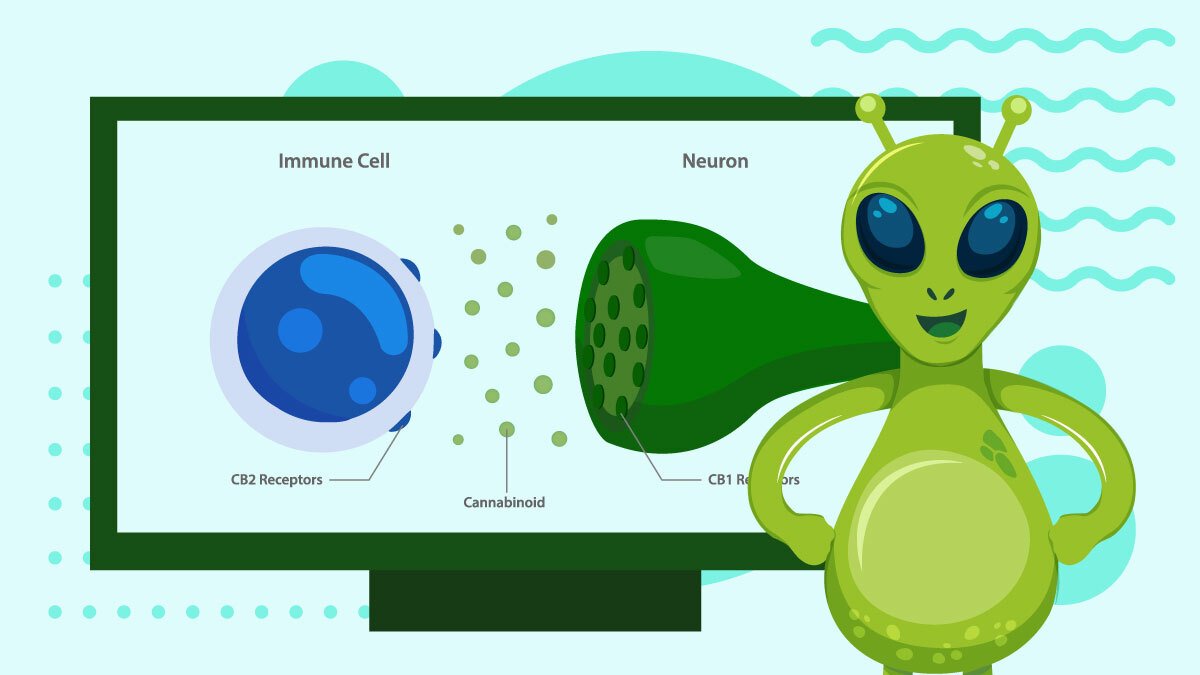
So far, only two receptors have been discovered and studied: CB1 and CB2. A possible third receptor, GPR55, is considered a type 3 because of its unique signaling system, thus opening up a new class of receptors.
Cannabinoids interact with these receptors in different ways. Some are agonists; they bind to the receptor and activate it. Others are antagonists and bind to the receptor but block its activation. Then, to keep everyone on their toes, you have rogue cannabinoids like cannabidiol (CBD) that have an obvious effect, but no one quite knows how.
Because even science isn’t an exact science.
While CB1 and CB2 impact most parts of the body, they have primary locations, which helps them fulfill specific roles.
CB1 Receptors
CB1 is primarily in the brain; it’s abundant in the neocortex, hippocampus, and cerebellum though it’s also in the basal ganglia, mostly in the SNr and segments of the globus pallidus. Activation of CB1 inhibits neuronal excitability and neurotransmission.
It’s also in peripheral organs and tissues like the reproductive system, endocrine glands, white blood cells, and spleen, among others.
As a refresher, because almost no one remembers high school biology, the neocortex is involved in high-order functions like rational thought and language:
- Hippocampus: It’s in the temporal lobe and has a huge part in learning and memory; it’s affected in many neurological and psychiatric disorders. The cerebellum controls complex motor skills and things like balance and equilibrium. It’s what helps you walk straight.
- Basal ganglia: Its primary function is motor control, executive functions, learned behavior, and emotions.
- Substantia Nigra: This supplies dopamine to the basal ganglia and nucleus accumbens, which are involved in reward, motivation, and addiction.
- SNR: This supplies dopamine to the basal ganglia and is involved in reward, motivation, and addiction.
- Globus pallidus: It’s inhibitory and suppresses unwanted movements.
Activating this receptor has benefits, but too much can cause changes in perception, emotion, balance, and overall brain function.
Remember, THC binds (strongly) to CB1 — you can probably see the connection by now. Next time you’re legally using weed, think of all the activity going on inside your head. That’ll keep you busy.
CB2 Receptors
These receptors are mainly in the immune system, though they’re also in the CNS, just to a lesser degree.
If you were to look, you’d find them in the major tissues that produce and regulate immune cells (such as the spleen and tonsils — yes, they have a purpose). These cells include mast cells, microglial cells, B and T lymphocytes, natural killer cells, monocytes, and macrophages.
Yes, this means another refresher. It’s important, or we wouldn’t do it:
- Mast cells: The first line of defense against infection. They’re located beneath the skin’s surface, throughout connective tissues, in nerves, around blood vessels, and in some mucosal surfaces. Their role is to mediate inflammatory responses and release chemical mediators, including histamine. Thank these for hypersensitivity (not emotional, that’s a different issue) and allergic reactions.
- Microglial cells: These make up 10% of all the cells in the brain, making them the most prominent immune cells in the CNS. When something goes wrong, these are the first to react. In fact, these cells seem to be involved in almost all brain diseases, from inflammation to strokes.
- B and T lymphocytes: They’re in the same family of natural killer cells; they’re all effector cells and part of the adaptive immune system — the group that reacts to a stimulus, creating an immune response. T cells destroy pathogens while B cells secrete antibodies, and all effector cells help eradicate cancer.
- Natural killer cells (NK cells): These cells sound violent, and in a sense, they are, but in a good way. They’re infamous for killing cells infected with a virus but might play a crucial role in pregnancy and can even spot early signs of cancer.
- Monocytes: They’re a kind of white blood cell produced in the bone marrow and can make up to 10% of the circulating white blood cells. They remove dead and damaged tissue and fight infections, cancer cells, and foreign substances.
- Macrophages: These cells search and destroy bacteria and other harmful organisms and initiate inflammation by activating other cells.
Part 2: The Endocannabinoids
There are two kinds of cannabinoids: endocannabinoids, those made by the ECS, and phytocannabinoids, those made by plants.
The two main endogenous cannabinoids are N-arachidonoylethanolamine or anandamide (AEA) and 2-arachidonoylglycerol (2-AG). We’ll focus on phytocannabinoids later — they deserve their own section.
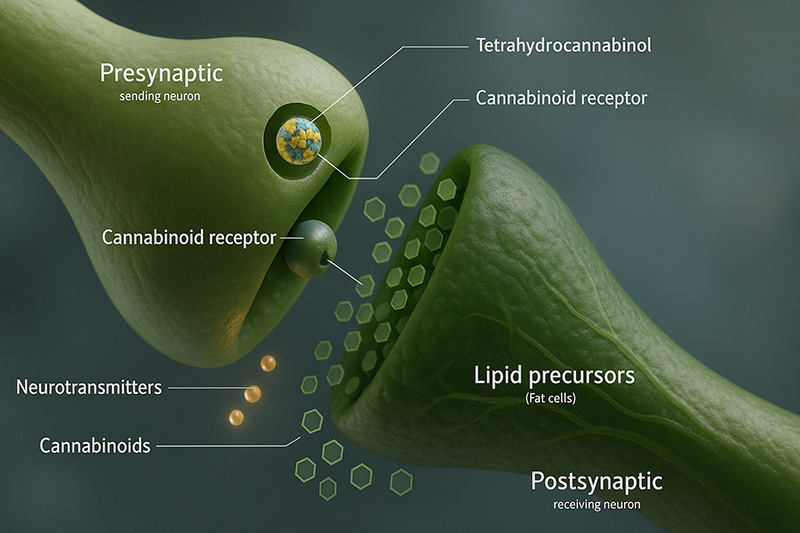
Endocannabinoids are found in tissue, organs, and bodily fluid and act as agonists of both CB1 and CB2.
Some seem to work as backward messengers; they’re made on-demand, released from postsynaptic neurons, and go back across the synapse to activate and alter the actions of the presynaptic cannabinoid receptors. This retrograde signaling is vital for specific kinds of synaptic plasticity, contributing to brain functions involving learning and memory.
Research shows that endocannabinoids have a vast influence on transmitter release, certain aspects of neuronal signaling, and actions involved with complex cognitive functions and processes.
AEA and 2-AG play similar roles, and both contain arachidonic acid, but different enzymes control how they’re made and broken down, and each one has a specific job.
2-AG
2-AG binds to both receptors, but more so with CB2, and seems to act as a neuromodulator. It’s a major source of arachidonic acid in the brain, liver, and lungs and plays a key role in prostaglandin synthesis.
Arachidonic acid helps protect the brain from oxidative stress and influences the growth and repair of neurons and skeletal muscle tissue. Prostaglandins are an important part of inflammation. These lipids control the blood flow and formation of blood clots in damaged tissues or infections.
Anandamide (AEA)
The endogenous cannabinoid anandamide is made by an enzyme called NAPE-PLD, a speicalized type of phospholipase D generating anandamide. Anandamide acts more like a stress-responsive modulator and binds strongly to CB1 receptors. This “bliss molecule” seems to be heavily involved in the brain’s reward circuitry and behavior reinforcement — so much so that researchers are studying it for its role in addiction and drug abuse.
Its depletion causes the opposite: feelings of fear, anxiety, and stress.
It doesn’t stop there — endocannabinoids don’t just bind to receptors. They also bind and activate TRPV1, a vanilloid receptor. Anandamide is also an agonist for some PPAR subtypes, a group of receptors that help regulate energy and metabolism homeostasis.
What are vanilloids, and why is TRPV1 special? Glad you asked.
Vanilloids
These compounds bind to the TRPV receptors, ion channels that respond to noxious stimuli (there are six receptors total if you’re curious). Noxious stimuli are something that can or will cause tissue damage.
The two most common vanilloids are vanillin (the primary component of the vanilla bean) and capsaicin (the component that gives chili peppers their heat) — two opposites from the same family.
Hopefully, you don’t have experience with another common one: nonivamide. That one is used in pepper spray.
TRPV1 Receptors
These are located mostly in nerve fibers, smooth muscle cells, and some vascular cells; they might even have a part in the health of the digestive, cardiovascular, and respiratory systems.
They specifically react to high temperatures, things with an acidic pH, and pretty much anything that could cause tissue damage.
TRVP1’s role with this kind of stimuli is extensive and has a domino effect. By transmitting signals to the nerve center, they activate signaling pathways in the cell, which causes a large-scale cellular response that regulates various related functions.
Remember that numbing burn that creeps to the back of your throat and continues through the digestive tract after eating a habanero? What about that intense pain that seeps into your lungs, crawls up your nostrils, and fills your eyes after getting sprayed with pepper spray? That’s all because of that vanilloid binding to the TRPV1 receptor.
Part 3: ECS Enzymes
Everything built must be destroyed, says physics. It equally applies to endocannabinoids.
Two main enzymes have this job; fatty acid amide hydrolase (FAAH) takes care of AEA while monoacylglycerol acid lipase (MAGL) deals with 2-AG. Cyclooxygenase-2 (COX-2) degrades both through oxidation, and there are a few others that help either break down or inactivate them.
Inhibiting these enzymes may have therapeutic potential — minus the issues that come with activating the CB1 receptor. For example, decreased anandamide hydrolase concentrations boost the anandamide levels, prolonging its beneficial effects on the human body.
The Role of the ECS For Your Health
It’s pretty clear the ECS impacts your health. In fact, it’s probably more accurate to say your health hinges on it.
We’ve peeked at a few of the small yet profound ways it influences our bodies, but what does that look like? What does it mean in layman’s terms?
The ECS helps maintain and balance most critical body systems, including:
- Central nervous system
- Peripheral nervous system
- Cardiovascular system
- Gastrointestinal system
- Reproductive system
- Skeletal system
- Immune system
- Metabolic processes
- Temperature regulation
- Memory
- Learning
- Appetite
- Stress response
- Pain sensation
- Mood
- Sleep
- Inflammation
- Pleasure and reward
- Motor control
Because the ECS affects so much, researchers are beginning to look at it for new ways to treat disease. Many health problems are connected to the ECS in some way.
Considering the ECS’ broad scope of influence, it only makes sense that varying extremes of endocannabinoids — whether they’re too high or too low, or too inactive or not active enough — could cause problems.
Clinical Endocannabinoid Deficiency (CED)
Though inconclusive, many studies back the theory that endocannabinoid levels, their production, metabolism, and abundance can result in Clinical Endocannabinoid Deficiency (CED) or ECS hyperactivity (overly high signaling).
An endocannabinoid system that is a little lax or slow at its job increases the risk for neurological diseases.
The ECS works closely with and even helps modulate neurotransmitters like serotonin and dopamine. Brain disorders like Alzheimer’s, Parkinson’s, and depression tend to coincide with low levels of neurotransmitters, like dopamine, serotonin, acetylcholine, and norepinephrine — all crucial to brain health.
The stress response can also be affected by an ECS that’s not functioning correctly. According to one theory, impaired signaling from either the ligands or the receptors might result in stress response activation or inappropriate stress adaption. Basically, it makes you feel excessive levels of stress or feel threatened when there’s no threat.
So far, it seems this kind of dysregulation comes from low levels of AEA. Endocannabinoids act as a buffer and help reduce and manage stress and anxiety. The AEA works to limit them through its actions in the amygdala. 2-AG is mobilized under exposure to repeat stressors or highly emotional states; it helps restrict the stress response and brings things back to normal.
ECS Hyperactivity
Hyperactive signaling of CB1 can suppress the appetite regulator and cause the over-consumption of food. Add that to enhanced reward circuits (again, CB1), and you get an unhealthy relationship with food.
It’s ok once in a while, like when the excessive signaling is because of THC, but scientists believe there’s a link between ECS hyperactivity, obesity, and eating disorders. Understanding how to influence CB1 could be a breakthrough for some of these issues.
Unfortunately, cancer cells tend to have elevated levels of endocannabinoids. The aggressiveness of cancer seems to be tied to the endocannabinoids’ concentration and metabolism.
The ECS: What Does the Research Say?
Because the ECS is so new and incredibly complex, it’s hard to tap into its potential right now. So far, there’s just a bunch of “what ifs” and theories that need more testing.
But that doesn’t make it any less exciting. There are no absolutes yet (there rarely are), but it’s hard to imagine the ECS not transforming medicine — it’s already changed our approach.
Tweaking the number of endocannabinoids or inhibiting their breakdown can drastically affect too many things to list, which brings another problem; it’s hard to trigger specific changes without setting off numerous others. Still, the possibilities are there.
We’ll take a more in-depth look at some of these, but here’s a list of health issues and diseases that might be treatable, or at least find relief, through the ECS:
- Neurodegenerative diseases
- Cancer
- Epilepsy
- Traumatic brain injury
- Obesity
- Multiple sclerosis
- Psychiatric disorders
- Drug and alcohol addiction
- Parkinson’s disease
- Huntington’s disease
- Spinal cord injury
- Cancer
- Stroke
- Hypertension
- Glaucoma
- Metabolic syndrome
- Osteoporosis
It’s an impressive list, hence the excitement.
What’s even more exciting? The “why” behind it. Don’t judge — some of us like this kind of thing. Take a look at what the research shows so far.
1. Cancer
The ECS is a target in the treatment of cancer, and its effects could be two-fold.
First, endocannabinoids show anticancer activity by slowing or stopping the increase and invasion of cancer cells and tumors. Second, studies show there are elevated levels of CB receptors and ligands in tumor tissue, and CB activation can counteract the formation of tumors.
There’s a connection between an altered ECS and cancer prognosis, outcome, and cancer type, hinting that it has a role in tumor growth and progression. The overexpression of the ECS coincides with tumor growth.
2. Neuroprotection
Cannabinoid receptor activation can help protect the brain and its functions in multiple ways.
Endocannabinoids can prevent microglial cell activation and decrease the amount of nitric oxide (NO) produced. Microglial cells influence antibacterial, antiviral, and antitumor activities by releasing NO and other proinflammatory mediators.
However, too much NO can cause brain injury and lead to neurodegenerative diseases and brain injury. Because of this, suppressing this activation could be a part of neurodegenerative disease treatments.
CB receptors, certain endocannabinoid enzymes, and ligands could help treat Traumatic Brain Injury (TBI) by altering neuroinflammation, excitotoxicity, and the death, structure, and remodeling of brain cells.
Other problems from TBI, such as learning and memory issues, motor impairment, and seizures, also responded to ECS manipulation.
3. Pain
If you exercise much, you’ll know there’s a point where it makes you feel really good — pain seems to diminish, or not matter. Runners know this as a “runner’s high.”
It turns out the ECS might be responsible for this, and the benefits go beyond that momentary bliss. Exercise boosts the levels of endocannabinoids, and some have pain-relieving properties. More importantly, there might be a connection between the ECS and how physical activity improves and influences the quality of life while aging.
That’s nice, but maybe you’re the type that thinks the words “exercise” and “feels good” shouldn’t be in the same sentence and cringe at the idea of breaking a sweat.
No judgment — you do you. Rest knowing your ECS takes care of other pain, too (including inflammatory pain); at least, studies point in that direction.
Endocannabinoids affect inflammation, acute and neuropathic pain, and immune regulation, making them a possibly potent analgesic.
4. Mental Disorders
The ECS’ job is to balance things, including emotional, cognitive, and neuropsychiatric functions. Deregulation on any level can lead to mental disorders, including schizophrenia, anxiety, and depression.
Stress adaptation is just one of the ways the ECS affects our mental state; it also helps regulate emotional memories. If it’s not functioning right, it could open the door for PTSD.
Some studies even link the central CB1 receptor, AEA, and 2-AG to depression and aggressive and suicidal behavior. It’s common for depressed and suicidal people to have abnormal levels of endocannabinoids in the prefrontal cortex (the part of the brain in charge of mood and impulsivity).
5. Obesity
There was a time we needed our bodies to store fat — now, not so much, at least for the majority of us. Yet, it still insists on watching out for us by making us desire high-calorie foods then storing those calories away for a rainy day. Those days never come, though, and the stored energy accumulates. Thanks, the endocannabinoid system.
Yes, the ECS is to blame for our food preferences and controls how it’s metabolized. Don’t use that as an excuse, though.
It does leave researchers hopeful that targeting the ECS can help treat obesity and metabolic diseases.
6. Medications & the ECS
We can see the possibilities, but how does this knowledge transfer to the development of medications that target the ECS?
Unfortunately, these medications would work by targeting endocannabinoids and inhibiting or increasing their binding to the receptors. Because the ECS has so much influence, it’s hard to find a way to impact one part without affecting other areas. On the other hand, these treatments would be quite safe.
CB1 antagonists could help treat obesity, metabolic issues, drug abuse, Parkinson’s, Alzheimer’s, inflammation, pain, osteoporosis, and some heart disease. CB2 antagonists could help with inflammation, dermatitis, and some neuroinflammatory disorders.
Currently, a few medications are available that work by directly impacting the ECS.
Cesamet and Marinol reduce nausea and vomiting in chemotherapy patients. Sativex helps with pain related to multiple sclerosis and cancer.
With more research and testing, hopefully, more will follow.
Phytocannabinoids & the ECS
Cannabis has been a staple throughout the ages — long before we knew the ECS existed. Humans knew of its effects and appreciated its therapeutic (and fun) benefits without knowing how it worked. Now that we’re more aware, we can appreciate it that much more.
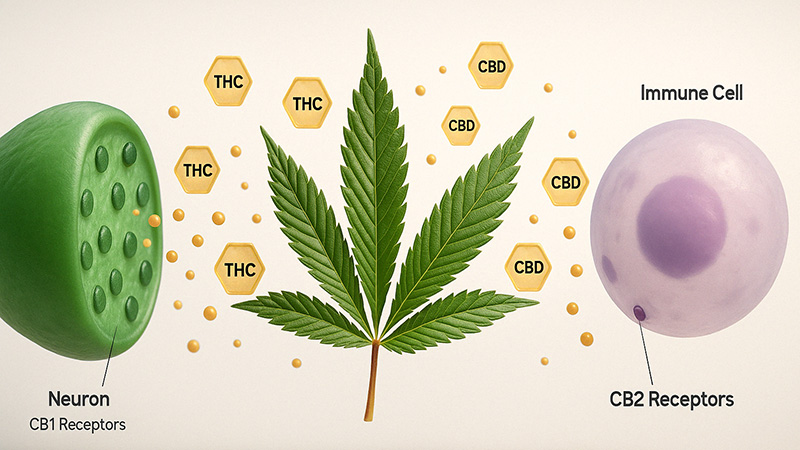
Phytocannabinoids or exogenous cannabinoids work similarly to endocannabinoids; they just come from cannabis instead of in-house. The two main cannabinoids, THC and CBD, act on the CB receptors either as antagonists or agonists and work as effectively, or nearly, as AEA and 2-AG. There are over 100 cannabinoids altogether, from what we know so far, though most remain unstudied. You can check out our recent post about the major cannabinoids found in cannabis plants to understand more of their uses and effects.
You’d think by now we would have massive studies on these complex compounds, but no. Every day brings something new, partly because cannabis remained unstudied for so long (legalities got in the way).
It wasn’t too long ago we thought phytocannabinoids were only in cannabis. Now, we know they’re also in the Rhododendron species, certain legumes, Radula marginata, and some fungi.
Each cannabinoid has a beneficial effect on the ECS (among other things), and each is unique.
The following phytocannabinoids are among the most popular:
THC (Delta 9 tetrahydrocannabinol)
THC is the most prevalent cannabinoid in cannabis and quite possibly the best known and most loved. Delta 9 THC is the primary active ingredient in marijuana that gives its psychoactive effects and plays a large part in its therapeutic benefits.
THC is a partial agonist and works by binding to both CB1 and CB2 receptors, but its bond is much tighter with CB1.
It’s also what separates federally legal hemp and federally illegal marijuana. The 2018 Farm Bill states all hemp products are permitted as long as they have less than 0.3% THC. However, plenty of states have legalized marijuana (more than 0.3% THC) for medical or recreational purposes, and others will probably join the club.
CBD (Cannabidiol)
Here’s another popular one, and it happens to be the other primary cannabinoid. It made history by being the first cannabinoid to be the main component of the FDA-approved medication, Epidiolex. This drug reduces seizures in rare forms of epilepsy.
CBD doesn’t bind to either receptor but influences them in some way. We’ll let you know as soon as the scientists have it figured out. Because it doesn’t bind to the CB1 receptor, it has no psychoactive properties. Instead, it tends to have calming effects.
CBC (Cannabichromene)
CBC is a major cannabinoid that binds loosely to both receptors but isn’t psychoactive. It also modulates TRPV1 receptors.
People mostly use it to reduce swelling and pain, though it can also be uplifting.
There are many others we could add, but research is limited. Just know that anecdotal evidence (and applying what we do know) points to a whole lot of potential.
Here are some of the other noteworthy cannabinoids:
- CBG (Cannabigerol)
- THCV (Tetrahydrocannabivarin)
- CBDV (Cannabidivarin)
- CBCV (Cannabichromevarin)
- CBGV (Cannabigerivarin)
The Entourage Effect
There’s one more aspect that has scientists both baffled and disagreeing over: the entourage effect. It’s the theory that the whole cannabis plant works best together, kind of like pieces to a puzzle that come together to make something spectacular. Again, it’s just a theory, but it’s backed by some research — and disapproved by some.
Cannabinoids alone seem to impact each other; add them with terpenes, and it’s a new ball game, or so they say. Terpenes are like cannabinoids because they work in similar ways but add their own unique twist with various effects.
Why does it matter? Maybe it doesn’t if you’re only using it recreationally. If you need it medically, it could mean a lot.
For example, CBD reduces the psychoactivity of THC. Other cannabinoids boost it. If you’re using these products for pain, you probably want to remain functional as well. That won’t happen if you’re staring at the TV, couch-locked, eating nachos.
Maybe scientists will verify the entourage effect and figure out how to harness its magical powers — it might be the key to targeting specific cannabinoids and creating particular results without the cascade of other effects.
A Brief History of the ECS
In 1988, while studying the effects of synthetic cannabinoids or artificial compounds that mimic THC, researchers noticed certain brain membranes seemed to have high-affinity binding sites for cannabinoids. Years later, they found DNA coding for this cannabinoid receptor and creatively called it “CB1.”
This study showed the receptor was located primarily in the central nervous system (CNS), which left unanswered questions, as most research does. THC doesn’t affect just the CNS, so they knew something was missing.
Researchers eventually found the answer in a second receptor primarily located in the immune system and, for the sake of consistency, named it “CB2.” Its location explained some of THC’s other, less prominent effects.
The next logical step was to look for the ligand (a molecule that binds to another molecule) that these receptors “receive” or attach to; being scientists, they knew there had to be an endogenous (internal) source. Sure enough, after many failed attempts and with a different perspective, scientists isolated a lipid cannabinoid-like component.
Because it acted similarly to THC, and to make things interesting, they named it “anandamide” after the Sanskrit word “ananda,” meaning “inner bliss.”
In the early 1990s, two independent studies found lipid fractions that also held cannabinoid activity. Some of these were structurally similar to anandamide, while others looked and acted completely different and were composed of sn-2-arachidonoyl-glycerol (2-AG).
Ultimately, they found 2-AG binds to both CB1 and CB2 receptors and is a full agonist to CB1.
The Current State of ECS Understanding
Fast forward thirty-some years, and we still have few details on this crucial internal setup that happens to control so much of our health.
There’s so little we know compared to what we still have to learn. Researchers are discovering new enzymes, receptors, and cannabinoid-related molecules every year, and it’s still unclear how much of it works and ties together.
What we do know only raises more questions and gives hope for a new way to treat many diseases, including ones that currently lack effective treatment.
Summary: A World of Possibilities
A “quick” summary of the ECS, its purposes, its possible benefits, other cannabinoids, and whatever else we covered?
Alright — the endocannabinoid system is a fairly new discovery, and no one knows much about it. It regulates most bodily systems like the immune system, mood, hunger, the cardiovascular system, and everything in between.
It consists of only three parts: receptors (CB1 and CB2), endocannabinoids (AEA and 2-AG), and enzymes that break down the endocannabinoids.
The most intriguing part of this all lies in cannabis and its close connection with the ECS. Who knew that this plant we’ve been using forever contains molecules that mimic the ones made by our bodies and carry so much influence on our health?
Testing is in the early phases, but the ECS is a possible target for treating many diseases. It’s hard to narrow down specific effects since the ECS impacts so much. One tweak could unintentionally set off many others.
Still, the ECS might be a new revolution in medication, and cannabis could find itself back in everyone’s favor. Time will tell.
FAQs About the Endocannabinoid System
The ECS plays an important role in the body and helps regulate many biological as well as physiological processes. This section answers some of the commonly asked questions about the ECS.
1. What is the Function of the Endocannabinoid System?
The main function of the ECS is to ensure there’s homeostasis or balance in the body’s chemical levels, any imbalance of which can result in symptoms or worsening of medical conditions.
2. What is the Function of the Cannabinoid Receptors?
The cannabinoid receptors are a specialized type of protein that the endogenous cannabinoids bind to to activate the ECS. Although found all over the body, the type 1 receptors are abundantly found in the brain and spinal cord and regulate biological and physiological processes like mood, memory, appetite, and pain, while the type 2 receptors are mostly located in the immune system and modulate immune response.
3. How Do You Activate the Endocannabinoid System?
The ECS is activated by the endogenous cannabinoids, which our cells produce in response to noxious stimuli like pain and stress. It can also be activated by phytocannabinoids, which is why we react strongly to the cannabinoids found in hemp or cannabis plant.
4. What Do Cannabinoids Do to the Body?
Exogenous cannabinoids like CBD and THC activate the ECS and influence bodily responses and functions, from pain and inflammation to fear and appetite. THC, in particular, binds very strongly to the type 1 receptor, producing the high associated with cannabis use.
5. What is the Role of the Endocannabinoid System in Pain?
The ECS plays a huge role in pain. It reduces pain perception by decreasing hypersensitivity to pain. It also reduces inflammation, the process of which releases pain-generating chemicals like substance P.
References Used
- Mouslech, Z., & Valla, V. (2009). Endocannabinoid system: an overview of its potential in current medical practice. Neuroendocrinol Lett, 30(2), 153-79. [1]
- Freund, T. F., Katona, I., & Piomelli, D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiological reviews. [2]
- Yang, H., Zhou, J., & Lehmann, C. (2016). GPR55–a putative “type 3” cannabinoid receptor in inflammation. Journal of basic and clinical physiology and pharmacology, 27(3), 297-302.
- Kendall, D. A., & Yudowski, G. A. (2017). Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Frontiers in cellular neuroscience, 10, 294. [4]
- Rosenbaum, T., & Simon, S. A. (2007). TRPV1 receptors and signal transduction. TRP ion channel function in sensory transduction and cellular signaling cascades, 69-84.
- Hashimotodani, Y., Ohno-Shosaku, T., & Kano, M. (2007). Endocannabinoids and synaptic function in the CNS. The Neuroscientist, 13(2), 127-137. [6]
- Freund, T. F., Katona, I., & Piomelli, D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiological reviews.
- Lu, H. C., & Mackie, K. (2016). An introduction to the endogenous cannabinoid system. Biological psychiatry, 79(7), 516-525.
- Scherma, M., Masia, P., Satta, V., Fratta, W., Fadda, P., & Tanda, G. (2019). The brain activity of anandamide: a rewarding bliss?. Acta Pharmacologica Sinica, 40(3), 309-323.
- Gunduz-Cinar, O., Hill, M. N., McEwen, B. S., & Holmes, A. (2013). Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends in pharmacological sciences, 34(11), 637-644.
- Rosenbaum, T., & Simon, S. A. (2007). TRPV1 receptors and signal transduction. TRP ion channel function in sensory transduction and cellular signaling cascades, 69-84.
- Lu, H. C., & Mackie, K. (2016). An introduction to the endogenous cannabinoid system. Biological psychiatry, 79(7), 516-525.
- van Egmond, N., Straub, V. M., & van der Stelt, M. (2021). Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annual Review of Pharmacology and Toxicology, 61, 441-463.
- Pacher, P., Bátkai, S., & Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological reviews, 58(3), 389-462.
- Russo, E. B. (2016). Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis and cannabinoid research, 1(1), 154-165.
- Hill, M. N., & Patel, S. (2013). Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of mood & anxiety disorders, 3(1), 1-14.
- Miękus, N., Olędzka, I., Harshkova, D., Liakh, I., Plenis, A., Kowalski, P., & Bączek, T. (2018). Comparison of three extraction approaches for the isolation of neurotransmitters from rat brain samples. International journal of molecular sciences, 19(6), 1560.
- Hillard, C. J. (2018). Circulating endocannabinoids: from whence do they come and where are they going?. Neuropsychopharmacology, 43(1), 155-172.
- Śledziński, P., Zeyland, J., Słomski, R., & Nowak, A. (2018). The current state and future perspectives of cannabinoids in cancer biology. Cancer medicine, 7(3), 765-775.
- Maroon, J., & Bost, J. (2018). Review of the neurological benefits of phytocannabinoids. Surgical neurology international, 9.
- Rodríguez de Fonseca, F., Del Arco, I., Bermudez-Silva, F. J., Bilbao, A., Cippitelli, A., & Navarro, M. (2005). The endocannabinoid system: physiology and pharmacology. Alcohol and Alcoholism, 40(1), 2-14.
- Pacher, P., Bátkai, S., & Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews, 58(3), 389-462.
- Fraguas‐Sánchez, A. I., Martín‐Sabroso, C., & Torres‐Suárez, A. I. (2018). Insights into the effects of the endocannabinoid system in cancer: a review. British journal of pharmacology, 175(13), 2566-2580.
- Portella, G., Laezza, C., Laccetti, P., De Petrocellis, L., Di Marzo, V., & Bifulco, M. (2003). Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. The FASEB Journal, 17(12), 1771-1773.
- Ramírez, B. G., Blázquez, C., del Pulgar, T. G., Guzmán, M., & de Ceballos, M. L. (2005). Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. Journal of Neuroscience, 25(8), 1904-1913.
- Waksman, Y., Olson, J. M., Carlisle, S. J., & Cabral, G. A. (1999). The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. Journal of Pharmacology and Experimental Therapeutics, 288(3), 1357-1366.
- Thameem Dheen, S., Kaur, C., & Ling, E. A. (2007). Microglial activation and its implications in brain diseases. Current medicinal chemistry, 14(11), 1189-1197.
- Schurman, L. D., & Lichtman, A. H. (2017). Endocannabinoids: a promising impact for traumatic brain injury. Frontiers in pharmacology, 8, 69.
- Watkins, B. A. (2018). Endocannabinoids, exercise, pain, and a path to health with aging. Molecular aspects of medicine, 64, 68-78.
- Barrie, N., & Manolios, N. (2017). The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. European journal of rheumatology, 4(3), 210.
- Starowicz, K., & Finn, D. P. (2017). Cannabinoids and pain: sites and mechanisms of action. Advances in Pharmacology, 80, 437-475.
- Ibarra-Lecue, I., Pilar-Cuéllar, F., Muguruza, C., Florensa-Zanuy, E., Díaz, Á., Urigüen, L., … & Callado, L. F. (2018). The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochemical pharmacology, 157, 97-107.
- Hill, M. N., Bierer, L. M., Makotkine, I., Golier, J. A., Galea, S., McEwen, B. S., … & Yehuda, R. (2013). Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology, 38(12), 2952-2961.
- Vinod, K. Y., & Hungund, B. L. (2006). Role of the endocannabinoid system in depression and suicide. Trends in pharmacological sciences, 27(10), 539-545.
- Pinna, G. (2021). Endocannabinoids and precision medicine for mood disorders and suicide. Frontiers in psychiatry, 12.
- Mazier, W., Saucisse, N., Gatta-Cherifi, B., & Cota, D. (2015). The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends in Endocrinology & Metabolism, 26(10), 524-537.
- Di Marzo, V. (2008). Targeting the endocannabinoid system: to enhance or reduce?. Nature reviews Drug discovery, 7(5), 438-455.
- Pertwee, R. G. (2009). Emerging strategies for exploiting cannabinoid receptor agonists as medicines. British journal of pharmacology, 156(3), 397-411.
- Maroon, J., & Bost, J. (2018). Review of the neurological benefits of phytocannabinoids. Surgical neurology international, 9. [39]
- Di Marzo, V., & Piscitelli, F. (2015). The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics, 12(4), 692-698.
- Maione, S., Piscitelli, F., Gatta, L., Vita, D., De Petrocellis, L., Palazzo, E., … & Di Marzo, V. (2011). Non‐psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. British journal of pharmacology, 162(3), 584-596.
- Nahler, G., Jones, T. M., & Russo, E. B. (2019). Cannabidiol and contributions of major hemp phytocompounds to the “entourage effect”; possible mechanisms. J. Altern. Complementary Integr. Med, 5.
- Finlay, D. B., Sircombe, K. J., Nimick, M., Jones, C., & Glass, M. (2020). Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Frontiers in pharmacology, 11, 359.
- Russo, E. B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. British journal of pharmacology, 163(7), 1344-1364.






